Read About MediSphere Success Stories
MediSphere Medical Research Center is excited to announce the F.D.A. Approval of the following drugs. These important milestones in delivering on the promise of new drugs to patients would not be possible without the cooperation of our patients who participated in the clinical research studies.
Thank you for your ongoing support.
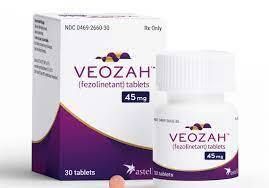
15 May, 2023
The FDA has just approved VEOZAH™ tablets for oral use for the treatment of moderate to severe (VMS) hot flashes and/or night sweats due to menopause. 60% to 80% of women experience these symptoms during or after the menopause transition and can have a disruptive impact on women’s daily activities and overall quality of life. VEOZAH™ is the first nonhormonal approved treatment.
By Medisphere Medical Research Center
•
23 Mar, 2022
• In clinical trials, RINVOQ (upadacitinib) achieved the primary endpoints of clinical remission (per modified Mayo Score [mMS]) at weeks 8 and 521-4 • A greater proportion of RINVOQ-treated patients achieved clinical response (per partial mMS [pmMS]) as early as week 2 and steroid-free clinical remission at one year, as well as key endoscopic and histologic improvement endpoints, at weeks 8 and 524 • First approved in 2019, RINVOQ is a JAK inhibitor approved for four indications across gastroenterology, dermatology and rheumatology4 NORTH CHICAGO, Ill., March 16, 2022 – AbbVie (NYSE: ABBV) today announced that the U.S. Food and Drug Administration (FDA) has approved RINVOQ® (upadacitinib) for the treatment of adults with moderately to severely active ulcerative colitis (UC) who have had an inadequate response or intolerance to one or more tumor necrosis factor (TNF) blockers. This FDA approval is the first indication for RINVOQ in gastroenterology and is supported by efficacy and safety data from three Phase 3 randomized, double-blind, placebo-controlled clinical studies. Experience the interactive Multimedia News Release here: https://www.multivu.com/players/English/8978351-abbvie-fda-ulcerative-colitis/ “There remains an unmet need for patients with moderately to severely active UC, who suffer from debilitating symptoms that are often unpredictable and burdensome,” said Thomas Hudson, MD, senior vice president of research and development, chief scientific officer, AbbVie. “With the approval of RINVOQ as a new treatment option, AbbVie continues our leadership in advancing research that can help impact the lives of people living with ulcerative colitis." The two induction studies (U-ACHIEVE and U-ACCOMPLISH) utilized RINVOQ 45 mg once daily for 8 weeks, and then 15 mg or 30 mg once daily for the maintenance study (U-ACHIEVE maintenance) through 52 weeks.1-4 Across all clinical trials, significantly more patients treated with RINVOQ achieved clinical remission at weeks 8 and 52, the primary endpoint based on the mMS: stool frequency subscore (SFS) ≤ 1 and not greater than Baseline, rectal bleeding subscore (RBS) = 0, endoscopy subscore (ES) of ≤ 1 without friability, compared to placebo. In addition, the studies met all ranked secondary endpoints, including endoscopic improvement and histologic-endoscopic mucosal improvement (HEMI), as well as corticosteroid-free clinical remission in the maintenance study. All primary and ranked secondary end- points achieved p-values of <0.001 versus placebo. 1-3 Dear Upa UC Investigators and Study Coordinators: The US FDA has approved Rinvoq (Upadacitinib) to treat adults with moderately to severely active ulcerative colitis who have had an inadequate response or intolerance to one or more tumor necrosis factor (TNF) blockers. We wanted to make sure you were aware of the approval as soon as possible. Please see the attached AbbVie Press Release for further information. The development and approval of RINVOQ in the US underscore AbbVie’s commitment to providing more treatment options for your patients living with UC. AbbVie has invested in UC for decades, with ongoing research designed to improve the lives of patients living with IBD. We set our expectations high, and continually seek to defy them. Thank you for the valuable contributions that you and your staff have made to this program! Sincerely, The AbbVie UPA UC Study Team Upadacitinib UC Program: U-Achieve, U-Accomplish & U-Activate M14-234 / M14-675 / M14-533

By MediSphere
•
17 May, 2018
The study drug Aimovig is now FDA Approved. MediSphere conducted this Migraine trial in 2015-2017. Welcome news to some of the millions of Americans who suffer from potentially debilitating migraine headaches! The new drug, Aimovig, was produced by Amgen and approved by the FDA on May 17, 2018. It is the first medicine in a new class that is designed to reduce the number of migraines among people who suffer them frequently. Aimovig is an expensive type of medication called a monoclonal antibody. It and several other migraine drugs in development, are based on research that begun in the 1980's. Antibodies are produced in living cells rather than in a chemical laboratory. People inject the drug themselves using a pen like device similar to those used for insulin. Amgen says it's ready to put the drug on the market within a week and has programs in place to help ease the cost to some patients. https://www.npr.org/sections/health-shots/2018/05/18/612296263/new-type-of-drug-to-prevent-migraines-heads-to-market

By MediSphere
•
19 Jan, 2017
Another successful trial at MediSphere Medical Research Center, LLC: The U.S. Food and Drug Administration (FDA) has approved Trulance™ (plecanatide) for the treatment of adults with chronic constipation of unknown cause (CIC). Trulance™ is the first drug designed to replicate a naturally occurring compound thought to stimulate fluid secretion in the gastrointestinal (GI) tract. Based on pre-clinical studies, this results in a stool consistency associated with more regular bowel function. * Synergy's plan is to make Trulance™ available in the U.S. later this quarter. (Excerpted from press release) The MediSphere team extends its deepest gratitude to all of our patients who participate in clinical trials that bring new medicines to market.
Address
Hours
Mon-Fri: 7:30am - 5:00pm
Sat-Sun: CLOSED